About Symposium
18th International EBHC Symposium 2023
Integrating evidence for enhanced outcomes
October 9-10, 2023 | hybrid format
Schedule | Sessions | Speakers | Partners | Conditions of participation | ![]() Preliminary Programme EN – 2 MB |
Preliminary Programme EN – 2 MB | ![]() Final Programme EN – 4 MB
Final Programme EN – 4 MB
The program of the 18th EBHC Symposium was carried out over 2 days within the following thematic blocks:
- Session 1: Opening of the Symposium
- Session 2: International HTA developement
- Session 3: Challanges for local governments – difficult choices, election time...
- Session 4: Poland and Ukraine – common healthcare challanges
- Session 5: The need of innovation in HTA
- Session 6: Best practices of integration in care
- Session 7: The value of medical care - the patient's perspective
Ever since the era of health technologies began, the process of generating scientific evidence has been dispersed, lengthy and divided into stages implemented independently by numerous teams. Initially, research design was too focused on optimising the scope of the collected data, as excessive data volumes could exceed a facility's capacities and its budget. It was necessary for researchers to consider costs (e.g., of analysts’ work), time (required calculations), technical limitations (database capacity) and silo mentality regarding research processes.
Integration no. 1 – uniform questionnaire formats
During the individual stages of the research process, data were formatted and processed to meet the requirements of various methods of analysis and inference. Significant technical, financial, and human resources were engaged in collecting, reproducing, sending, and storing paper and later electronic research documentation. Data gathered with such effort were (and still are) closely guarded by various institutions. Creating and protecting sensitive data is important not only from the perspective of the average patient, but also due to the matter of intellectual property. In the meantime...
Integration no. 2 – scale, assessment, credibility...
Conducting isolated studies, even those large-scale ones, is impractical in the long run, as it does not allow for reaching synergy by combining results from numerous examined populations. Secondary research offered the opportunity to create more accurate conclusions, but it required a standardisation of analytical processes including methods of data synthesis for scientific goals, creating new technologies, as well as analysing healthcare systems.
Integration no. 2.5 – reimbursement!
Another revolution was brought about by applying scientific evidence to assessments of the validity of reimbursing health technologies. The progress of medicine, supported by the pharmaceutical industry (and its primary and secondary research) and, to varying degrees, by the public sector resulted in an enormous growth of knowledge resources, as well as difficulties in implementing them in health care and improving the quality of patient care. The subjective needs of patients were quantified in health technology assessment or quality assessment processes more and more often; however, the adopted tools would not provide an answer to the questions regarding satisfying the needs of people covered by healthcare. top
Integration no. 3 – human factor
New concepts emerged as a counterweight for these imperfections; they were supposed to complete and support HTA-based decision-making and health care management. They include, among others, Value-Based Health Care (VBHC), which focuses on patients’ needs, and Real-World Evidence (RWE). The need to include patients, who are the broadest group of stakeholders begins to take shape in provisions and regulations concerning patients’ participation in the decision-making processes (e.g., NICE's Public Involvement programme). RWE proposes using mass real-world data already at the lowest decision-making processes (e.g., by physicians), bypassing the time-consuming processing and formal reports drawn up by institutions. The COVID-19 pandemic and development of digitalisation accelerated the use of RWE. At the same time, it was the catalyst which boosted the process of creating RWE. Doctors fighting with the pandemic were updating the entire world on an ongoing basis about the results of their activities. That was the first mass use of RWE – without a formal system and with the use of improvised measures (social media, phones, emails).
As technology progresses, especially the ubiquity of digitalisation and a huge increase of database possibilities, it is easier for the contemporary researchers to process data. They do not need to be afraid of the abundance of data. Gathering different types of data resembles playing with blocks which have different types of connection combinations. The more connections our blocks have, the larger the possibility of attaching them to the “structures” (research) of other researchers. However, as the blocks get larger, we need increasingly bigger boxes to store them (databases). In return, we get the possibility to integrate scientific evidence acquired from completely different sources, environments, and research techniques. Creating new connections become the subject of international regulations e.g., of the European Health Data Space (EHDS). top
Integration no. 4 – just new toys or already a matrix?
Integrating scientific evidence at the HTA level becomes increasingly popular and is often initiated at very early stages of creating health technologies. An approach combining randomised controlled trials (RCT) and real-world evidence can serve as a strategic path for growth for all stakeholders of the healthcare system. These new possibilities will also bring about new challenges, not only in the context of medical advances, but also in the evolution of healthcare systems.
The sudden growth of the amount of collected data might give raise to concerns whether their reasonable analysis is possible, but in fact the answer might lie in the implementation of artificial intelligence (AI). Perhaps AI will soon allow us to determine the key models for analysing data and assessing efficacy, e.g., without the need to conduct randomised controlled trials (RCT). It will surely help choose patients for such trials more accurately. Thanks to the RWE analysis with the AI support, it might be possible to create a digital model of the healthcare system that will facilitate following diagnostic paths, monitoring areas that require interventions, identifying, and breaking though diagnostic barriers. Such a model could also adjust the manufacture levels to the system's current needs or recommend the optimal number of places at medical universities based on the trend analysis.
Digitalisation creates such huge opportunities that finding and implementing reasonable ideas to their full potential will still need to take some time. However, new dilemmas are already emerging, such as issues regarding privacy and safety of citizens’ data and the necessity to create solutions that do not generate further inequalities in healthcare.
I o tym wszystkim dyskutowaliśmy na 18. Międzynarodowym Sympozjum EBHC. top
Venue
Kosciuszko Mound in Krakow
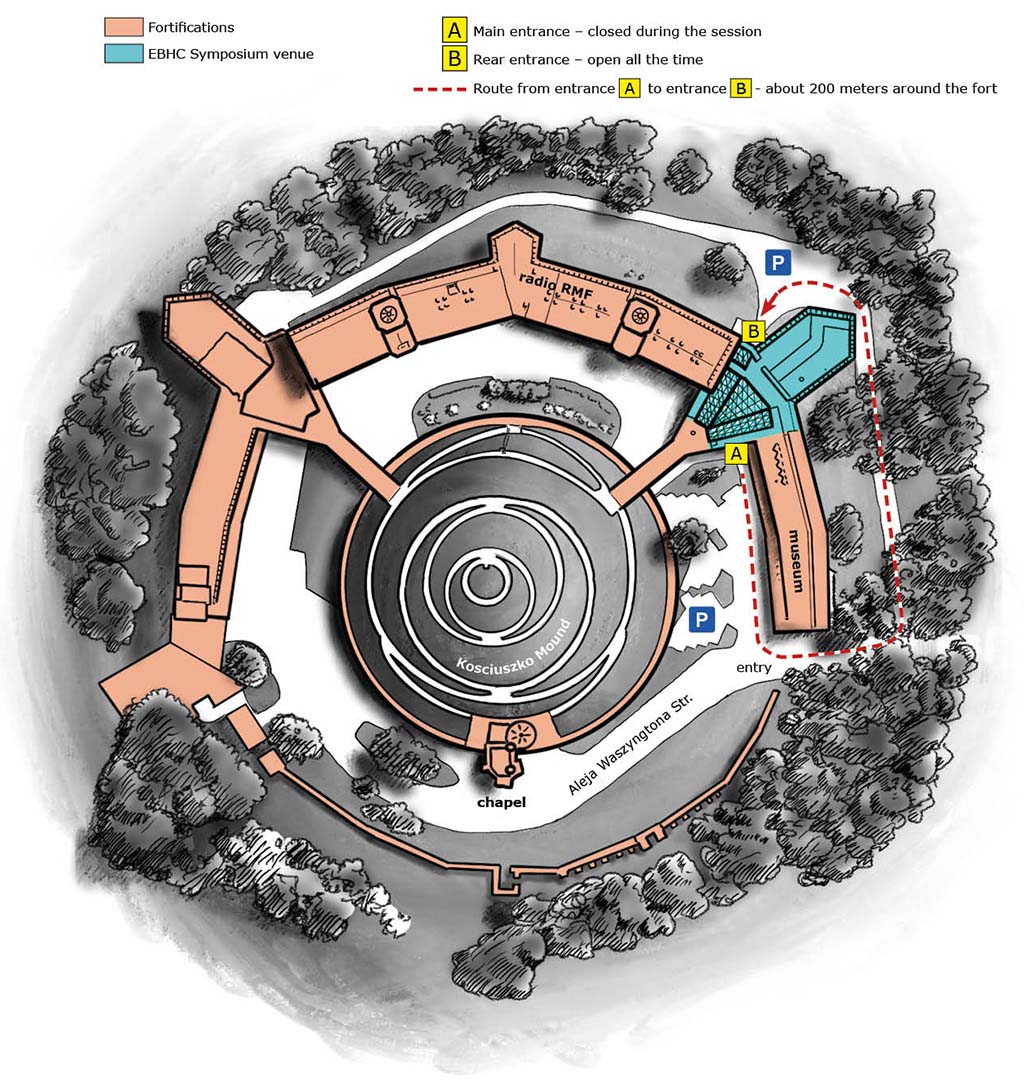
In 2023, we organized a Symposium on Kosciuszko Mound – a unique place, associated with the history of Krakow for 200 years. The symposium was held in a conference space built in the fort's courtyard under a glass roof. The catering space was located in the fort buildings.
The venue of the EBHC 2023 Symposium is a conference and event center where conferences are systematically held and has conference facilities enabling the exchange of scientific and medical information and the transfer of knowledge. The property does not have any recreational facilities. As part of the EBHC Symposium, conference organizers and participants didn't have access to any tourist facilities and attractions. It is a separate conference center with a separate entrance, separated from the tourist attraction (Kościuszki Mound).
Kosciuszko Mound in Krakow, Kraków, al. Waszyngtona 1
Symposium in hybrid format - how does it work

- on-line participation (sessions)

- text chat for asking questions
- chat with other participants
- access to session recordings
- You decide when to watch
Personal participation in the Symposium, apart from all elements of online participation, gives access to the Symposium spaces.
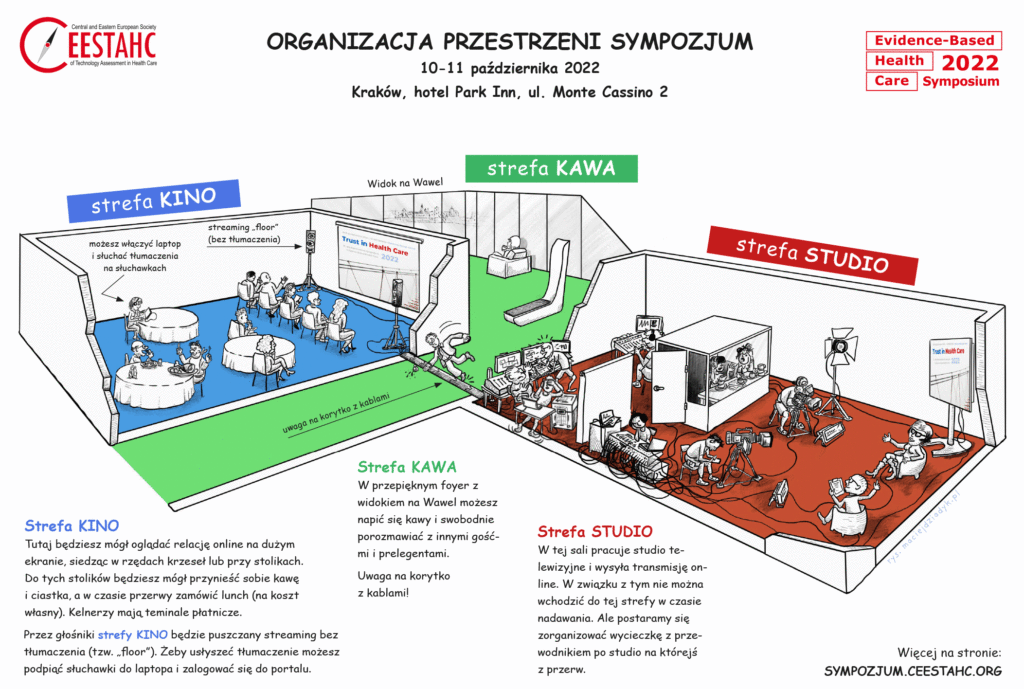
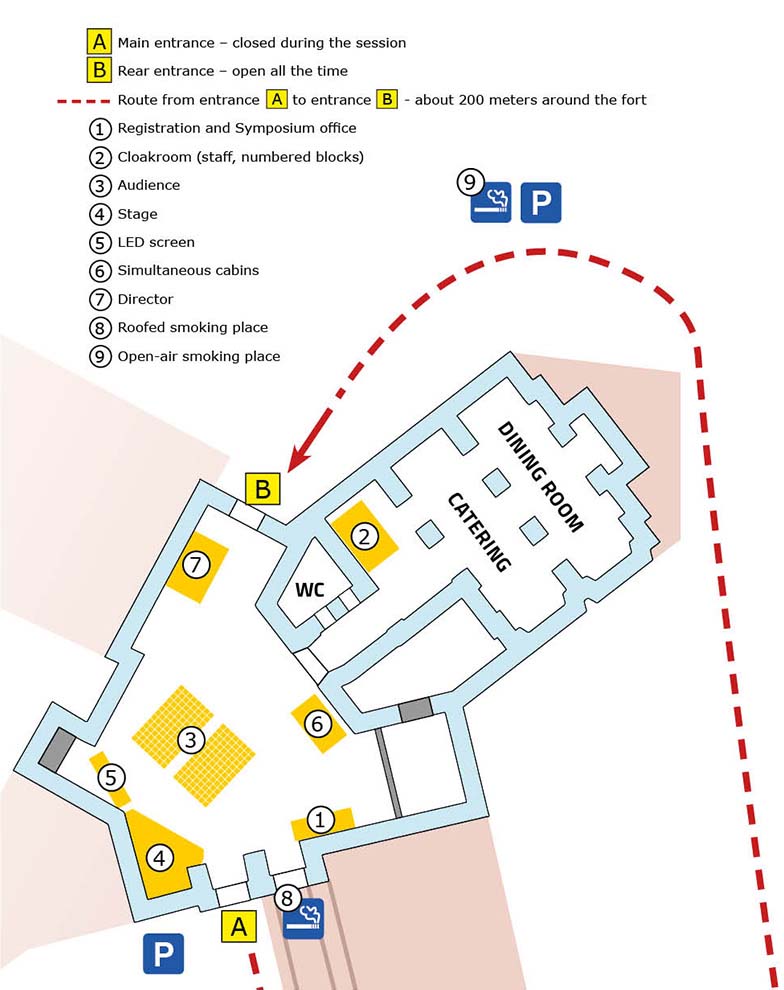
CINEMA space
Here you will be able to watch the online coverage on the big screen, sitting in rows of chairs or at tables. You will be able to bring coffee and biscuits to these tables, and order lunch during the break (at your own expense). Waiters have payment temptation.
There will be no translation played through the speakers of the CINEMA space. To hear the translation, you can connect the headphones to your laptop and login to the portal.
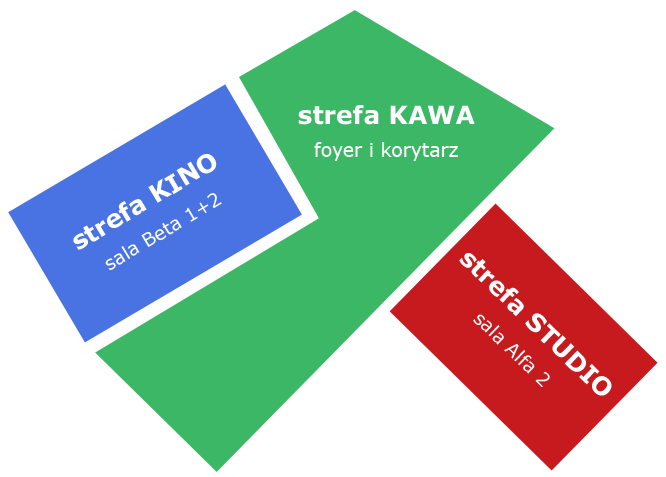
CAFFEE space
In the beautiful foyer overlooking the Wawel Castle, you can drink coffee and talk freely with other guests and speakers.
STUDIO space
Our TV studio works in this room and sends an online broadcast. Therefore, you cannot enter this space during sessions. But we will try to arrange a guided tour of the studio during one of the breaks.